We serve 1-Bromo-3-methylbutane CAS:107-82-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
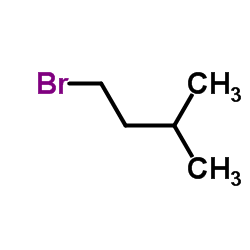
Contact us for information like 1-Bromo-3-methylbutane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Isobutylmethylbromide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Methylbutylbromid Use and application,1-Bromo-3-methylbutane technical grade,usp/ep/jp grade.
Related News: In August, the Company announced that the U.S. Patent and Trademark Office issued U.S. Patent No. 10,370,452 covering compositions and uses of effector T cells expressing a CAR, where such T cells are derived from a pluripotent stem cell, including an iPSC.3,4-Difluorophenol manufacturer The purpose of APIs according to the FDA is to cause ‘pharmacological activity or other direct effects in the diagnosis, cure, mitigation, treatment or prevention of disease or to affect the structure and function of the human body.Pyridoxine dipalmitate supplier With nearly 194 billion US dollars of original research medicines facing the expiration of patents in the past five years, more and more domestic companies are focusing on the corresponding characteristic APIs, and have started research and development and production preparations in advance. The scale of production and export of APIs will continue to expand and grow.Dimethyl nonanedioate vendor With nearly 194 billion US dollars of original research medicines facing the expiration of patents in the past five years, more and more domestic companies are focusing on the corresponding characteristic APIs, and have started research and development and production preparations in advance. The scale of production and export of APIs will continue to expand and grow.Oligomannate, which uses extract from marine brown algae as raw material, received a conditional green light to treat mild-to-moderate level AD, the National Medical Products Administration (NMPA) said in a statement on its website late on Saturday.

