We serve 1-Bromohexane CAS:111-25-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
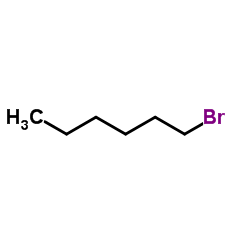
Contact us for information like 1-Bromohexane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Hexane,1-bromo- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,HEXYLBROMIDE Use and application,1-hexylbromide technical grade,usp/ep/jp grade.
Related News: Social media users — including numerous medical experts — questioned whether the findings were supported by clinical evidence from treating coronavirus patients.2-fluoro-4-methyl-3-nitropyridine manufacturer Social media users — including numerous medical experts — questioned whether the findings were supported by clinical evidence from treating coronavirus patients.2,4-Diaminoanisole sulfate supplier Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).(S)-(-)-3-(Acetylthio)-2-methylpropionic Acid vendor Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).Since first being reported in the city of Wuhan, where it is believed to have originated at a seafood market, the virus has not provoked unusual symptoms in people who have been diagnosed.

