We serve 1,1-Dimethylurea CAS:598-94-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
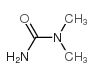
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | White to off-white crystalline powder | Conform |
| Assay | ≥98.0% | 98.27% |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like 1,1-Dimethylurea chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Urea, N,N-dimethyl- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,1-Dimethylurea Use and application,1,1-DIMETHYLUREA technical grade,usp/ep/jp grade.
Related News: That’s four days earlier than it had initially planned. Delta’s last China-bound flight left on Saturday, February 1, and its final returning flight from China to the United States leaves on Sunday. N-[1-Benzyloxymethyl-2-phenylsulfanyl-eth-(E)-ylidene]-N’-(2,4-dinitro-phenyl)-hydrazine manufacturer Large pharmaceutical companies are investing a disproportionately large amount of annual revenue in research and development (R&D), according to GlobalData’s Company Financials database.Acetic acid, [ethoxy(4-methylphenyl)phosphinyl]-, ethyl ester supplier Large pharmaceutical companies are investing a disproportionately large amount of annual revenue in research and development (R&D), according to GlobalData’s Company Financials database.2-(3′,4′-difluorophenyl)pyridine vendor Because API companies usually adopt a different synthetic route from the original research company, compared with the drug itself, the impurities contained in the generic drug API have not been verified by the original drug for many years of use and require more careful testing and control.Because API companies usually adopt a different synthetic route from the original research company, compared with the drug itself, the impurities contained in the generic drug API have not been verified by the original drug for many years of use and require more careful testing and control.

