We serve 2-(4-bromophenyl)-4-phenylquinazoline CAS:540466-42-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
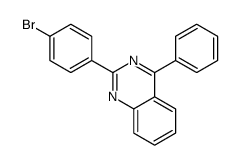
Contact us for information like 2-(4-bromophenyl)-4-phenylquinazoline chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-(4-Bromophenyl)-4-phenylquinazoline physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Quinazoline,2-(4-bromophenyl)-4-phenyl Use and application,BDPQL technical grade,usp/ep/jp grade.
Related News: He added that the government raised the travel advice warning to level four, the highest, meaning they’re now advising people not to travel to mainland China. The warning aims to address “the issue of the human-to-human transmission of the coronavirus.”1-Bromo-4-chloronaphthalene manufacturer Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added “The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.4-(methylsulfanyl)phenol supplier Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added “The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.2-Deoxy-L-ribose vendor Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added “The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.

