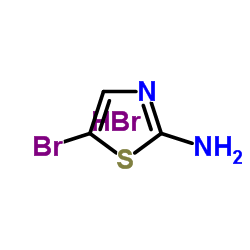
We serve 2-Amino-5-bromothiazole monohydrobromide CAS:61296-22-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | Light yellow to light brown crystalline powder | Conforms |
| Purity | ≥96.0% | 96.7% |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like 2-Amino-5-bromothiazole monohydrobromide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Thiazolamine, 5-bromo-, monohydrobromide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Amino-5-bromothiazole monohydrobromide Use and application,2-Amino-5-bromothiazole monohydrobromide technical grade,usp/ep/jp grade.
Related News: Also that day the CDC reported the first case of person-to-person transmission in Illinois between household members.7-Fluoro-6-nitro-4-hydroxyquinazoline manufacturer Also that day the CDC reported the first case of person-to-person transmission in Illinois between household members.[1,1′-Binaphthalen]-2-ol, 2′-[2-methyl-1-(1-methylethyl)propoxy]- supplier The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses.4-[2-(3,4,5-Trifluoro-benzyl)-oxazol-5-yl]-benzenesulfonyl chloride vendor But many others apparently didn’t need confirmation. Posts on Weibo, China’s Twitter-like platform, purportedly showed people lining up at night outside pharmacies across China to buy Shuanghuanglian, ironically going against the authorities’ advice to avoid congregating in public.Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).

