We serve 2-Chloro-3,5-dinitropyridine CAS:2578-45-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
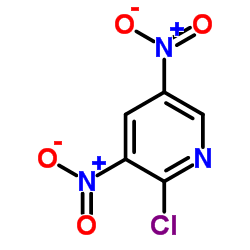
Contact us for information like 2-Chloro-3,5-dinitropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3,5-dinitro-2-chloropyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Chloro-3,5-dinitropyridine Use and application,2-Chloro-3,5-dinitropyridine technical grade,usp/ep/jp grade.
Related News: Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.4-Tert-Butylbenzenesulfonamide manufacturer South Korea and Japan are barring noncitizens who traveled recently to Hubei, the province at the center of the outbreak.2'-Deoxy-2'-fluorouridine supplier South Korea and Japan are barring noncitizens who traveled recently to Hubei, the province at the center of the outbreak.4-Bromocumene vendor The second is known as the excipient, which is the inactive substance that serves as the vehicle for the API itself.The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances.

