We serve 2-Chloro-5-methyl-3-nitropyridine CAS:23056-40-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
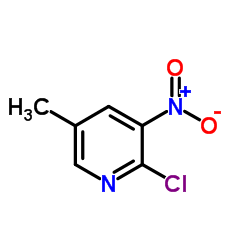
Contact us for information like 2-Chloro-5-methyl-3-nitropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-chloro-3-nitro-5-methylpyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Chloro-5-Methyl-3-Nitro Pyridine Use and application,2-Chloro-5-methyl-3-nitropyridine technical grade,usp/ep/jp grade.
Related News: During the deadly SARS pandemic of the early 2000s, another traditional Chinese herbal medicine, Banlangen, made from the root of a flowering woad plant and used to treat the common cold, was sold out in many pharmacies due to a popular but clinically unproven belief that it could help prevent SARS, which is closely related to the Wuhan coronavirus.4-Amino-2-picoline manufacturer At the same time, through years of imitation and advanced technology learning, more and more domestic companies have participated in the field of highly original and characteristic APIs.2-(2,2,2-Trifluoroethoxy)phenol supplier As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.2-amino-N-(5-chloropyridin-2-yl)-5-methoxybenzamide vendor As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.

