We serve 2-Chlorophenylboronic acid CAS:3900-89-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
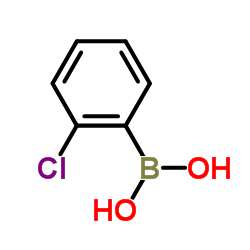
Contact us for information like 2-Chlorophenylboronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Chlorophenyl-dihydroxyborane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,ortho-chlorophenylboronic acid Use and application,2-Chlorophenylboronic acid technical grade,usp/ep/jp grade.
Related News: Zhejiang Huahai Pharmaceutical: It is a leading company in domestic APIs, especially in the field of cardiovascular drugs.2-Biphenylboronic acid manufacturer The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.(E)-3-(4-Amino-3,5-dimethylphenyl)acrylonitrile hydrochloride supplier The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.1,2,3-Trifluoro-4-methylbenzene vendor DPH and BPHC continue to work closely with the CDC to maintain vigilance during this virus outbreak.The Philippines, the United States and Australia have expanded travel restrictions, temporarily barring noncitizens who have recently traveled to China.

