We serve 2-Ethoxyethylamine CAS:110-76-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
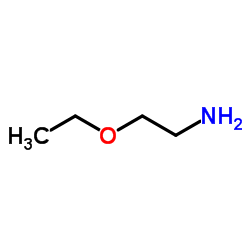
Chemical Name: 2-Ethoxyethylamine
CAS.NO:110-76-9
Molecular Formula:C4H11NO
Molecular Weight:89.13620
Synonyms:
2-EthoxyethylaMine
O-Ethylethanolamine
2-ethoxyethanamine
Ethanamine, 2-ethoxy-
2-Aminoethyl Ethyl Ether
Physical and Chemical Properties:
Density: 0.9±0.1 g/cm3
Boiling point: 108.8±13.0 °C at 760 mmHg
Melting point: /
Flash point: 17.7±8.6 °C
Refractive index: 1.409
Specification:
Appearance:Colorless transparent liquid
Purity:≥99.0%
Moisture Content: 0.5%
Impurity: 0.3%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application:Applicationd for production of herbicide.
Contact us for information like 2-Ethoxyethylamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Aminoethyl Ethyl Ether physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Ethoxyethylamine Use and application,O-Ethylethanolamine technical grade,usp/ep/jp grade.
Related News: Cases recorded in Thailand, Taiwan, Germany, Vietnam, Japan, France and the United States involved patients who had not been to China.2',3',5'-Tri-O-acetyl-2-fluoroadenosine manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.4-Bromo-2,3-difluorobenzaldehyde supplier “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.4-(4-chlorobutyl)pyridine,hydrochloride vendor Australia has started denying entry to all travelers who have come from or transited through mainland China.Beta Bionics, Inc. recently announced it has received Breakthrough Device designation from the US FDA for its investigational iLet Bionic Pancreas System.

