We serve 2-Ethyl-3,5-dimethylpyrazine CAS:13925-07-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
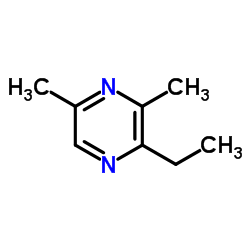
Contact us for information like 2-Ethyl-3,5-dimethylpyrazine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-ethyl-3,5-dimethylpyrazine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-ethyl-3,5-dimethylpyrazine Use and application,2-ethyl-3,5-dimethylpyrazine technical grade,usp/ep/jp grade.
Related News: At present, there are more than 8,000 domestic API manufacturers, but they mainly produce bulk APIs with low technical content.4-nitrobenzene-1,3-diamine,sulfuric acid manufacturer Both patients were being treated in isolation units at a Manila hospital.3-Chloro-2-cyanopyridine supplier The StartScore model was developed by the company’s in-house supply chain experts based on actual performance data.2-Bromobutyric acid methyl ester vendor The upstream industry for the R & D, production and sales of pharmaceutical intermediates is the basic chemical raw material industry, and the downstream industry is the chemical bulk drug and chemical pharmaceutical preparation industry.ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.

