We serve (2-Methoxynaphthalen-1-yl)boronic acid CAS:104116-17-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
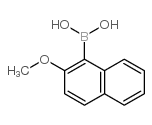
Contact us for information like (2-Methoxynaphthalen-1-yl)boronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Methoxynaphtalene-1-boronic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,21MONBA Use and application,(2-Methoxynaphthalen-1-yl)boronic acid technical grade,usp/ep/jp grade.
Related News: The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.2-(2-Chloroethoxy)-Benzenesulfonamide manufacturer Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers.3,3-Pentamethylene Glutarimide supplier The South Korean government is banning entry to all foreign nationals who visited China’s Hubei province in the past 14 days.Benzenesulfonyl Chloride vendor The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.The target screening performed by Retrogenix will focus on hundreds of novel, prioritized antibodies in Resonant’s collection.

