We serve 2-(Trifluoromethoxy)benzoic acid CAS:1979-29-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
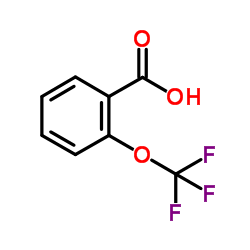
Contact us for information like 2-(Trifluoromethoxy)benzoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-TtrifluoroMethoxybenzoicacid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-TtrifluoroMethoxybenzoicacid Use and application,2-TtrifluoroMethoxybenzoicacid technical grade,usp/ep/jp grade.
Related News: The Company’s first-of-kind approach involves engineering human iPSCs in a one-time genetic modification event and selecting a single engineered iPSC for maintenance as a clonal master iPSC line.2,6-Difluoropyridine manufacturer Health officials said it remained unclear where he had developed the disease.L-Homoserine supplier Health officials said it remained unclear where he had developed the disease.4-fluorobutyl acetate vendor High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.

