We serve 2,3-dichloro-5-fluoropyridine CAS:185985-40-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
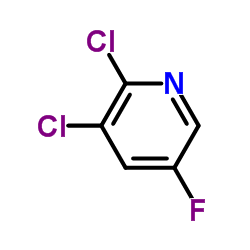
Contact us for information like 2,3-dichloro-5-fluoropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,ABBYPHARMA AP-31-5779 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,AC-5997 Use and application,ABBYPHARMA AP-31-5779 technical grade,usp/ep/jp grade.
Related News: That impacts airlines’ business, making it less financially attractive for them to fly those routes to China.2-Bromo-11,11-dimethyl-11H-benzo[b]fluorene manufacturer That impacts airlines’ business, making it less financially attractive for them to fly those routes to China.1-Tetralone supplier Pharmaceutical intermediates and APIs belong to the fine chemical industry.DL-Histidine Monohydrochloride Monohydrate vendor Pharmaceutical intermediates and APIs belong to the fine chemical industry.Refers to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs or innovative drugs). It mainly meets the needs of international original research drug companies and emerging biopharmaceutical companies for innovative drugs at various stages of clinical research, registration approval and commercialization of drugs. Contains advanced intermediates used in the manufacture of this drug substance that need to be regulated by regulatory authorities.

