We serve (2,4-Dichloro-5-isopropoxyphenyl)hydrazine CAS:40178-22-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
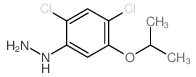
Contact us for information like (2,4-Dichloro-5-isopropoxyphenyl)hydrazine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,4-Dichloro-5-(1-methylethoxy)phenylhydrazine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,4-Dichloro-5-(1-methylethoxy)phenylhydrazine Use and application,(2,4-Dichloro-5-isopropoxyphenyl)hydrazine technical grade,usp/ep/jp grade.
Related News: 1,4-Dichlorobutane manufacturer With the speeding up of review and approval, the number of approved innovative drugs in China is about to usher in a year of blowout.L-Menthyl glyoxylate hydrate supplier With the speeding up of review and approval, the number of approved innovative drugs in China is about to usher in a year of blowout.3-Amino-2-Chlor-6-Methylphenol vendor Drug manufacturers make medicines by mixing APIs and pharmaceutical excipients.Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.

