We serve 2,5-Dichloropyridine CAS:16110-09-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
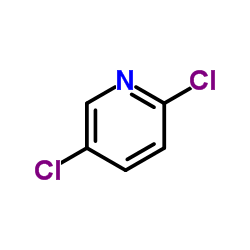
Contact us for information like 2,5-Dichloropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,5-dichloro pyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,5-dichloro pyridine Use and application,2,5-Dichloropyridine technical grade,usp/ep/jp grade.
Related News: Such was the apparent demand sparked by the notice that the compound formula sold out on some stores on China’s e-commerce platform Taobao.4-Amino-L-phenyl-N-phthalylalanine ethyl ester manufacturer Such was the apparent demand sparked by the notice that the compound formula sold out on some stores on China’s e-commerce platform Taobao.4-Bromotoluene supplier Targets within the tumor microenvironment may include both plasma membrane proteins as well as proteins typically secreted into the extracellular space.3-triethoxysilyl-N-(3-triethoxysilylpropyl)propan-1-amine vendor Cases recorded in Thailand, Taiwan, Germany, Vietnam, Japan, France and the United States involved patients who had not been to China.“Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.

