We serve (2S,3R)-3-Amino-2-hydroxy-4-phenylbutyric acid CAS:59554-14-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
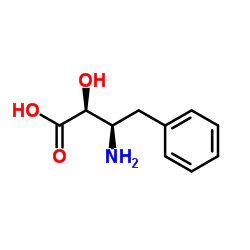
Chemical Name: (2S,3R)-3-Amino-2-hydroxy-4-phenylbutyric acid
CAS.NO: 59554-14-2
Synonyms:
3-Amino-2-Hydroxy-4-Phenylbutyric Acid
Molecular Formula: C10H13NO3
Molecular Weight: 195.21500
Physical and Chemical Properties:
Density: 1.285 g/cm3
Boiling point: 428.5ºC at 760 mmHg
Melting point: /
Flash point: 213.0±28.7 °C
Refractive index:1.595
Specification:
Appearance: white powder
Purity:≥99.0%
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:
Storage:2°C-8°C.Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application:
Intermediates of Ubenimex CAS:58970-76-6.
Contact us for information like (2S,3R)-3-Amino-2-hydroxy-4-phenylbutyric acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Amino-2-Hydroxy-4-Phenylbutyric Acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Amino-2-Hydroxy-4-Phenylbutyric Acid Use and application,3-Amino-2-Hydroxy-4-Phenylbutyric Acid technical grade,usp/ep/jp grade.
Related News: Any drug or medication is composed of two components.cumene manufacturer In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.(3S)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid supplier In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.L-Histidine Monohydrochloride Monohydrate vendor In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.In 2004, the National Development and Reform Commission, the former Ministry of Health, and the Ministry of Finance jointly formulated and promulgated the Administrative Measures for the Configuration and Use of Large Medical Equipment. This method uses X-ray electronic computer tomography (CT) devices and medical magnetic resonance imaging equipment, digital subtraction angiography X-ray machines of 800 mA or more as the management products of large-scale medical equipment, Purchase and use need to be reported to the provincial health administrative department for approval before approval. However, in recent years, with the comprehensive coverage of medical insurance, such basic medical facilities are being widely popularized, and the relevant implementation rules have directly linked the number of equipment to the number of doctors. Therefore, as the demand for imaging equipment continues to increase, it will have a greater boosting effect on the upstream contrast agent and its raw material market.

