We serve 3-Acetyl-2,5-dichlorothiophene CAS:36157-40-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
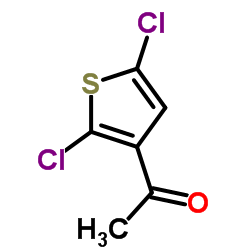
Chemical Name: 3-Acetyl-2,5-dichlorothiophene
CAS.NO:36157-40-1
Synonyms:
1-(2,5-Dichlorothiophen-3-yl)ethanone
Ethanone, 1-(2,5-dichloro-3-thienyl)-
Molecular Formula: C6H4Cl2OS
Molecular Weight:195.06600
Physical and Chemical Properties:
Density: 1.452g / cm3
Boiling point: 120-122 ° C 4mm
Melting point: 37-40 ° C (lit.)
Flash point:> 230 ° F
Refractive index: 1.575
Specification:
Appearance: White to pale yellow crystalline solid
Purity:≥98%
Moisture Content: ≤0.5%
Impurity: ≤0.5%
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:Stored in a cool and dry well-closed container. Keep away from moisture and strong light/heat.
Application:Intermediates of Brinzolamide CAS:138890-62-7
Contact us for information like 3-Acetyl-2,5-dichlorothiophene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ethanone, 1-(2,5-dichloro-3-thienyl)- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Ethanone, 1-(2,5-dichloro-3-thienyl)- Use and application,Ethanone, 1-(2,5-dichloro-3-thienyl)- technical grade,usp/ep/jp grade.
Related News: The unit of the British drugmaker, which is challenging HIV drug market leader Gilead Sciences, said it will work with the FDA to determine the next steps for the new drug application.2-Methoxyphenothiazine manufacturer The unit of the British drugmaker, which is challenging HIV drug market leader Gilead Sciences, said it will work with the FDA to determine the next steps for the new drug application.hexamethyldisiloxane supplier He was the partner of a 38-year-old Chinese woman who was travelling with him. She was the first confirmed case reported in the Philippines.2-Trifluoromethoxyphenol vendor Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added “The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.

