We serve 3-Bromo-5-chloropicolinonitrile CAS:760207-83-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
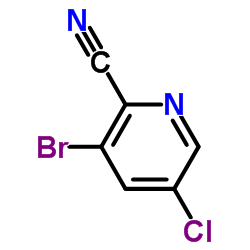
Contact us for information like 3-Bromo-5-chloropicolinonitrile chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Bromo-2-cyano-5-chloropyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Bromo-5-chloropicolinonitrile Use and application,3-bromo-5-chloropyridine-2-carbonitrile technical grade,usp/ep/jp grade.
Related News: Taking Teva as an example, it has 2 large R & D centers and 3 professional R & D centers in 5 countries, which effectively supports the development of Teva’s generic pharmaceutical sector.(E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole manufacturer Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.3H-1,3,4-thiadiazole-2-thione supplier Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.(S)-1,2,3,4-Tetrahydro-1-Naphthoic Acid vendor Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.The latest outbreak comes as China grapples with an African swine fever epidemic that has infected tens of thousands of pigs. It could stoke more worries among its people about the country’s food supply.

