We serve 3-Bromo-5-methylpicolinic acid CAS:1211515-68-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
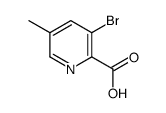
Contact us for information like 3-Bromo-5-methylpicolinic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-bromo-5-methylpyridine-2-carboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Bromo-5-methylpicolinic acid Use and application,3-bromo-5-methylpyridine-2-carboxylic acid technical grade,usp/ep/jp grade.
Related News: As proof-of-principle for the unique advantages arising from selecting a single engineered iPSC clone for the production of CAR T-cell therapy, the scientists assessed 747 clones after engineering a pool of cells using CRISPR. It was found that only about 2% of clones met the Company’s standards for overall quality including containing both bi-allelic disruption of the TCR, proper insertion of the CAR into the TRAC locus without random transgene integrations, and no evidence of off-target genomic modifications or translocations.Sodium L-Ascorbyl-2-Phosphate manufacturer As many as 9,000 medical workers in Hong Kong have pledged to strike this week, a threat that alarms the territory’s officials as they are struggling to contain the coronavirus outbreak.3,4-Diethoxyaniline supplier Characteristic APIs represented by cardiovascular, antiviral, antitumor and other categories are targeted at generic APIs with patent expiring original research drugs, which have high scientific and technological content and rich profits. They have developed in China in the past decade. The more active API segment has contributed to the rise of Huahai Pharmaceutical, Chuangnuo Pharmaceutical, and Jiangbei Pharmaceutical.2-Bromo-3-fluorotoluene vendor U.S. drugmaker Gilead dominates the HIV market and it will keep up the pressure with fast-growing Biktarvy, which was approved early last year.What’s the background?

