We serve 3-(Chloromethyl)pentane CAS:4737-41-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
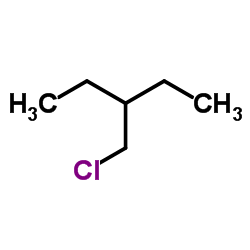
Chemical Name: 3-(Chloromethyl)pentane
CAS.NO: 4737-41-1
Molecular Formula: C6H13Cl
Molecular Weight: 120.62000
Synonyms:
2-Aethyl-butylchlorid
2-ethyl-1-chlorobutane
3-chloromethylpentane
1-chloro-2-ethylbutane
Pentane, 3-(chloromethyl)-
1-Chlor-2-aethyl-butan
Pentane,3-(chloromethyl)
3-Chlormethyl-pentan
Physical and Chemical Properties:
Density: 0.891 g / mL at 25ºC (lit.)
Boiling point: 126-128ºC753 mm Hg (lit.)
Flash point: 66 ° F
Refractive index: n20 / D 1.423 (lit.)
Specification:
Appearance: Colorless Liquid
Purity:≥98.0%
Water:≤0.1%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application: used in organic synthesis.
Contact us for information like 3-(Chloromethyl)pentane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Chlormethyl-pentan physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Aethyl-butylchlorid Use and application,2-Aethyl-butylchlorid technical grade,usp/ep/jp grade.
Related News: Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application.2-methyl-5-(trifluoromethyl)aniline manufacturer Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application.1,1,1,3,3,3-hexafluoropropan-2-ol supplier Britons being brought from Wuhan to the UK are being placed in quarantine at Arrowe Park Hospital in the Wirral, northwest England, Raab said.3-chloropyridine-2-carboxylic acid vendor Britons being brought from Wuhan to the UK are being placed in quarantine at Arrowe Park Hospital in the Wirral, northwest England, Raab said.In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.

