We serve 3-fluoro-1H-pyridin-4-one CAS:22282-73-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
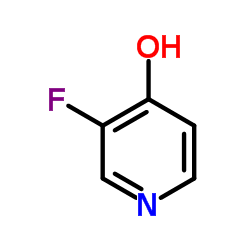
Contact us for information like 3-fluoro-1H-pyridin-4-one chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Fluoro-4-pyridinol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-fluoro-4-hydroxypyridine Use and application,3-Fluoro-4-pyridinol technical grade,usp/ep/jp grade.
Related News: The government is leveraging its large patient population to push non-domestic drugs companies to cut prices to their lowest level globally.B-(10-[1,1'-Biphenyl]-3-yl-9-anthracenyl)boronic acid manufacturer The government is leveraging its large patient population to push non-domestic drugs companies to cut prices to their lowest level globally.3-(N-Methylpentylamino)Propionic Acid Hydrochloride supplier “Once these risks have been identified then a project team can be put together combining the necessary expertise to provide the integrated services and tailored support to ensure continuity of supply from beginning to end.”2-chloro-6-(ethylamino)-4-nitrophenol vendor This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?

