We serve 3-Fluoro-4-nitrobenzoic acid CAS:403-21-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
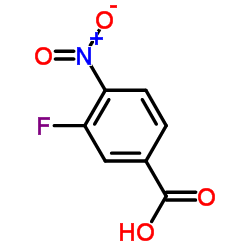
Contact us for information like 3-Fluoro-4-nitrobenzoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Benzoic acid, 3-fluoro-4-nitro- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Fluoro-4-nitrobenzoic acid Use and application,Benzoic acid, 3-fluoro-4-nitro- technical grade,usp/ep/jp grade.
Related News: Countries and territories that have confirmed cases: Thailand, Japan, Hong Kong, Singapore, Taiwan, Australia, Malaysia, Macau, Russia, France, the United States, South Korea, Germany, the United Arab Emirates, Canada, Britain, Vietnam, Italy, India, the Philippines, Nepal, Cambodia, Sri Lanka, Finland, Sweden and Spain.2-Chloro-N-(2-ethyl-6-methylphenyl)acetamide manufacturer This is very visible in herbal medicines in which the API is frequently a combination of several mixtures and/or substances which when used together cause pharmacological activity on the body.2,2'-[5-(Hydroxymethyl)-1,3-phenylene]bis(2-methylpropanenitrile) supplier From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.2-Bromopropionyl chloride vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.Here’s what Apple told CNN Business in a statement:

