We serve 3-phenylmethoxypyridin-2-amine CAS:24016-03-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
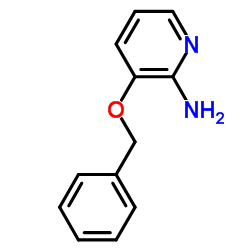
Chemical Name: 3-phenylmethoxypyridin-2-amine
CAS.NO:24016-03-3
Synonyms:
2-AMINO-3-BENZYLOXYPYRIDINE,BUFF COLOURED SOLID
3-benzyloxy-pyridin-2-ylamine
2-Amino-3-benzyloxypyridine
3-benzyloxy-2-aminopyridine
Molecular Formula: C12H12N2O
Molecular Weight: 200.23600
Physical and Chemical Properties:
Density: 1.18 g/cm3
Boiling point:342ºC
Melting point: 92-94 °C(lit.)
Flash point: 172.6ºC
Refractive index: 1.622
Specification:
Appearance:White to light yellow crystalline powder
Purity:≥99%
Water:0.5% max
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:Stored in a cool and dry well-closed container. Keep away from moisture and strong light/heat.
Application:Intermediates of Paliperidone CAS:144598-75-4
Contact us for information like 3-phenylmethoxypyridin-2-amine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-benzyloxy-2-aminopyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-benzyloxy-pyridin-2-ylamine Use and application,3-benzyloxy-2-aminopyridine technical grade,usp/ep/jp grade.
Related News: The workers are demanding that Hong Kong close all border checkpoints to visitors from mainland China, saying they represent a threat to health care workers in the city.chloromethyl carbonochloridate manufacturer The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.3-(1-Aminoethyl)phenol supplier The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.4-Fluoro-2-methylbenzoic acid vendor “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.“Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.

