We serve 3,4-Dimethyl-1,2-cyclopentanedione CAS:13494-06-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
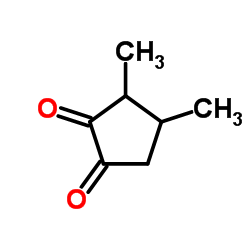
Contact us for information like 3,4-Dimethyl-1,2-cyclopentanedione chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3,4-Dimethyl-cyclopentan-1,2-dion physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3,4-DIMETHYL-1,2-CYCLOPENTADIONE Use and application,2-Cyclopentanedione,3,4-dimethyl-1 technical grade,usp/ep/jp grade.
Related News: Multinational drugmakers usually cut the price of drugs when they go off-patent and face competition from generic versions, but such price drops were slow to happen in China, partially because many local drugmakers were unable to develop high-quality generic drugs to compete with off-patent branded drugs.4'-Methoxyacetoacetanilide manufacturer Large pharmaceutical companies are investing a disproportionately large amount of annual revenue in research and development (R&D), according to GlobalData’s Company Financials database.(S)-(+)-3-Hydroxytetrahydrofuran supplier In terms of business, the industrial business increased by 7.97% year-on-year, while the revenue from the trading business shrank to a certain extent. The growth rate of industrial business was significantly slower than 24% in the first half of the year, showing that the growth rate of industrial business was lower in the third quarter, which was also the main reason for the decline in the company’s operating income in the third quarter. The company’s industrial business is mainly pesticide intermediates, accounting for about 85%. The scale of the pesticide intermediate business is large and the base is high, which is putting pressure on growth.9-Fluorenylmethyl pentafluorophenyl carbonate vendor Professor Coleman was involved in some of the first research into MERS in the US.In recent years, China’s bulk drug companies have gradually completed the upgrade of the product structure of bulk raw materials to specialty raw materials and intermediates. The industry’s leading companies have further developed the research and development layout of high-barrier generic pharmaceutical raw materials with multiple patents that have not yet expired.

