We serve 3′,5′-Difluoroacetophenone CAS:123577-99-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
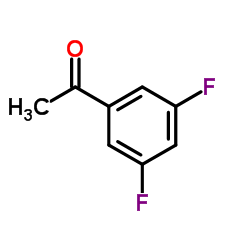
Contact us for information like 3′,5′-Difluoroacetophenone chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3,5-Difluoroacetophenone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-(3,5-difluorophenyl)ethanone Use and application,1-(3,5-difluorophenyl)ethanone technical grade,usp/ep/jp grade.
Related News: Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.cuminaldehyde manufacturer Chinese nationals coming from China and connecting through another foreign airport will be denied travel. Those with pre-clearance are exempted.Methyl Acetimidate Hydrochloride supplier In addition, although the API and intermediate industries are capital-intensive and technology-intensive industries, new competitors are still joining.2-FLUORO-5-METHYLBENZOIC ACID vendor The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.“Ultimately, this is a public health decision,” she said, adding that the restrictions were precautionary measures to keep the country virus-free and contain the worldwide outbreak.

