We serve 3,5-Difluoropyridine-2,6-diamine CAS:247069-27-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
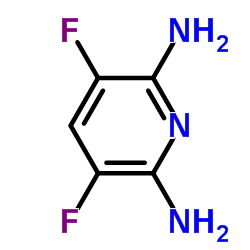
Contact us for information like 3,5-Difluoropyridine-2,6-diamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,6-Diamino-3,5-difluoropyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3,5-Difluoropyridine-2,6-diamine Use and application,3,5-Difluoro-pyridine-2,6-diamine technical grade,usp/ep/jp grade.
Related News: The main API products include antihypertensive, psychotropic and anti-AIDS special APIs. Antihypertensive APIs are mainly pulic and sartan drugs, and they are the world’s major suppliers of pulic and sartan APIs.3,5-Dimethyl-4-hydroxybenzonitrile manufacturer Chinese President Xi Jinping vowed to tackle the spread of the virus, saying: “People’s lives and health should be given top priority and the spread of the outbreak should be resolutely curbed.”3,4-Dimethylaniline supplier Only when the drug substance is processed into a pharmaceutical preparation can it become a drug for clinical application.O-ethylhydroxylamine,hydrochloride vendor In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.There are also new directives that relate to US citizens.

