We serve 4-Bromo-3-fluoroanisole CAS:408-50-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
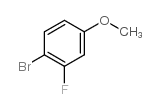
Contact us for information like 4-Bromo-3-fluoroanisole chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Bromo-3-fluoroanisole physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-Bromo-3-fluoroanisole Use and application,4-Bromo-3-fluoroanisole technical grade,usp/ep/jp grade.
Related News: A number of UK citizens had trouble reaching the muster point in time for the first flight out because of road blocks and other restrictions in place in Wuhan.1,2,3,4-Tetrahydro-1,1,4,4,6-pentamethylnaphthalene manufacturer For example, an active ingredient to relieve pain is included in a painkiller.thymidine supplier According to statistics, in 2017, China’s total production of chemical drugs reached 3.478 million tons, a year-on-year increase of 1.6%. The main business income showed a steady increase, from 328.972 billion yuan in 2012 to 573.475 billion yuan in 2017. The total profit was 48.644 billion yuan, but profit margins remain low (8.48% in 2017).1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butane-1,3-dione vendor Green Valley said it would launch the drug “very soon” in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.Dr. John K. Westwick, CEO of Resonant, added “Resonant has created a platform for rapid generation of novel anti-tumor antibody candidates.

