We serve 4-Cyanopyridine CAS:100-48-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
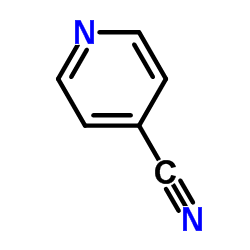
Chemical Name:4-Cyanopyridine
CAS.NO: 100-48-1
Synonyms:
Isonicotinic acid nitrile
pyridine-4-carbonitrile
Isonicotinonitrile
4-Pyridinecarbonitrile
Molecular Formula: C6H4N2
Molecular Weight: 104.10900
Physical and Chemical Properties:
Density: 1.12g / cm3
Boiling point: 196 ° C
Melting point: 76-79 ° C (lit.)
Flash point: 88 ° C
Refractive index: 1.539
Specification:
Appearance: White Crystalline Powder
Purity:≥99.0%
Water:0.5% Max.
Sulfated ash:0.1% Max.
Heavy metals:10ppm Max
Total Impurity:0.2% Max.
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application:
Intermediates of Topiroxostat CAS:577778-58-6.
Contact us for information like 4-Cyanopyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Pyridinecarbonitrile physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,pyridine-4-carbonitrile Use and application,Isonicotinic acid nitrile technical grade,usp/ep/jp grade.
Related News: In order to find answers to these questions, our staff in the development department set about conducting a series of experiments.5-methyl-6,7-dihydro-5H-cyclopenta[b]pyrazine manufacturer Speaking to Sky News, Raab said he could not confirm the “precise number” of those arriving into the UK on Sunday.7-chloro-1-heptanol acetate supplier Speaking to Sky News, Raab said he could not confirm the “precise number” of those arriving into the UK on Sunday.N-OCTYL ACRYLATE vendor A GSK spokesman said it was not yet clear by when ViiV would be able to address the FDA’s concerns.A GSK spokesman said it was not yet clear by when ViiV would be able to address the FDA’s concerns.

