We serve 4-(Difluoromethyl)benzonitrile CAS:55805-10-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
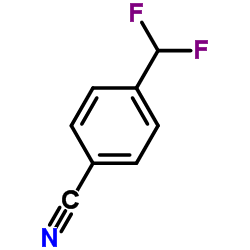
Contact us for information like 4-(Difluoromethyl)benzonitrile chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-(Difluoromethyl)benzonitrile physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-(Difluoromethyl)benzonitrile Use and application,4-(Difluoromethyl)benzonitrile technical grade,usp/ep/jp grade.
Related News: INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.11α-Hydroxy Canrenone manufacturer In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.4-Dibenzothienylboronic acid supplier In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.Pentyl chloroformate vendor In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.Judging from the geographical distribution of enterprises, the largest distribution of API companies is Jiangsu and Zhejiang, with more than 300 enterprises; followed by Shandong, Sichuan and Hubei.

