We serve 4-Fluoro-3-methylaniline CAS:452-69-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
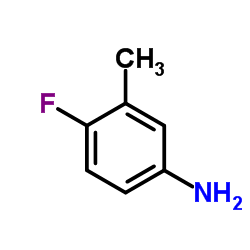
Contact us for information like 4-Fluoro-3-methylaniline chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Fluoro-m-toluidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-Amino-2-fluorotoluene Use and application,5-Amino-2-fluorotoluene technical grade,usp/ep/jp grade.
Related News: Beijing will strengthen its monitoring of overseas drug market and collect global prices for imported medicines, Friday’s guideline said.(S,S)-2,8-Diazabicyclo[4,3,0]Nonane manufacturer Beijing will strengthen its monitoring of overseas drug market and collect global prices for imported medicines, Friday’s guideline said.4-Bromo-2-fluorobenzyl bromide supplier This part is mainly anti-tumor drugs, prostate drugs and certain hormone drugs.2-Bromo-3-Fluoro-4-Picoline vendor Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS.Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS.

