We serve 4-Fluoronitrobenzene CAS:350-46-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
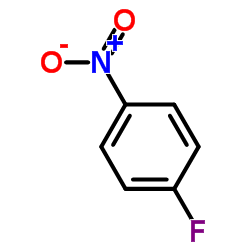
Contact us for information like 4-Fluoronitrobenzene chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-Fluor-4-nitrobenzol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,P-FLUORONITROBENZENE Use and application,P-FLUORONITROBENZENE technical grade,usp/ep/jp grade.
Related News: Bulk bulk medicines: overcapacity, low prices, future capacity and output will be reduced, and it is possible to transfer foreign production.4-tert-Butylbenzylamine manufacturer ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.4-(trifluoromethyl)phenol supplier It’s the first confirmed Wuhan coronavirus death from outside of mainland China, and brings the total toll to 305.N,N-dimethyl-1,4-phenylenediamine vendor With the global industrial division of labor and the change in the business model of multinational pharmaceutical companies, the outsourced market for patented drug substances will further expand.“Inceptua Medicines Access is delighted to be selected as Onconova’s partner for the Pre-approval Access Program for rigosertib.

