We serve 4-Methoxyphenylboronic acid CAS:5720-07-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
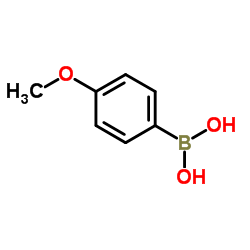
Contact us for information like 4-Methoxyphenylboronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,para-methoxyphenylboronic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-Anisylboronic acid Use and application,para-methoxyphenylboronic acid technical grade,usp/ep/jp grade.
Related News: The new restrictions on foreign nationals begin on February 3, Prime Minister Jacinda Ardern announced in a press release Sunday.3-Chlorobenzyl cyanide manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.O-(6-Chlorobenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium Tetrafluoroborate supplier Social media users — including numerous medical experts — questioned whether the findings were supported by clinical evidence from treating coronavirus patients.5-Fluoropicolinic acid vendor The downstream industry of the pharmaceutical intermediate industry is mainly the production of APIs, and the relationship between APIs and preparations is in the upstream and downstream industry chain. The consumer demand for downstream preparations will directly affect the demand for APIs.Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

