We serve 4-phenylbutyric acid CAS:1821-12-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
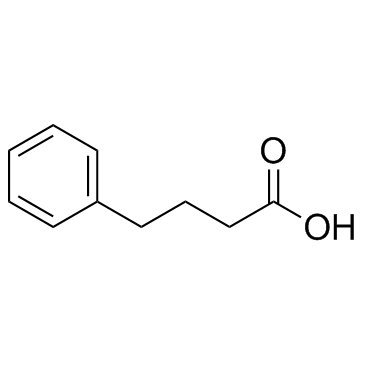
Contact us for information like 4-phenylbutyric acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Benzenebutyric acid; physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-phenylbutyric acid Use and application,4-phenylbutyric acid technical grade,usp/ep/jp grade.
Related News: The national airlines of Egypt, Qatar and Saudi Arabia have halted flights to and from China.methyl cyanoacetate manufacturer The Company’s immuno-regulatory product candidates include ProTmune, a pharmacologically modulated, donor cell graft that is currently being evaluated in a Phase 2 clinical trial for the prevention of graft-versus-host disease, and a myeloid-derived suppressor cell immunotherapy for promoting immune tolerance in patients with immune disorders. Fate Therapeutics is headquartered in San Diego, CA.(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium (2R)-hydroxy(phenyl)ethanoate supplier Bulk drug substances mainly include vitamins, antibiotics, hormones, antipyretics and analgesics, among which vitamin C, erythromycin thiocyanate, penicillin, azithromycin, and semi-synthetic cephalosporins are the most concentrated products in the industry in terms of production and overcapacity.2-Anilino-6-dibutylamino-3-methylfluoran vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.

