We serve 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine CAS:145783-15-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
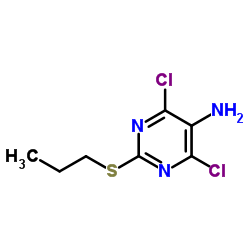
Product Name: 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Synonym: Intermediate of Ticagrelor; 5-Amino-4,6-dichloro-2-(propylthio)pyrimidine; 4,6-dichloro-2-(propylsulfanyl)-5-pyriMidinaMine
Cas No.: 145783-15-9
Formula: C7H9Cl2N3S
MW: 238.14
EINECS No.: 808-051-8
HS Code: 2942000000
UN No.: 1759
Hazard class: 8 categories
Packing level: Class III
Specification
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | Yellow to yellowish brown oily matter or solid |
Conforms |
| Single impurity | ≤0.5% | 0.17% |
| Total impurity | ≤1.0% | 0.28% |
| Water | ≤0.5% | 0.15% |
| Assay | 98.0%-102.0% | 99.75% |
| Conclusion | Conforms to Factory Standard | |
Application
Intermediate of Ticagrelor.
Packaging
25 kg/barrel, can also be packaged according to customer requirements.
Storage
Store in a cool, ventilated warehouse.
Keep away from fire and heat.
The temperature should not exceed 37 °C.
Contact us for information like 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4,6-dichloro-2-(propylsulfanyl)-5-pyriMidinaMine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4,6-dichloro-2-(propylsulfanyl)-5-pyriMidinaMine Use and application,Intermediate of Ticagrelor technical grade,usp/ep/jp grade.
Related News: From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.4-(1-phenylethyl)benzene-1,3-diol manufacturer From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.2-(2-aminopropan-2-yl)-N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-6-oxopyrimidine-4-carboxamide supplier From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.Isopropyl isocyanate vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.

