We serve 4,6-difluoro-1H-indole-2-carboxylic acid CAS:247564-66-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
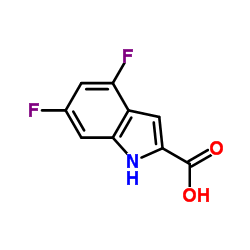
Contact us for information like 4,6-difluoro-1H-indole-2-carboxylic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4,6-Difluoroindole-2-carboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4,6-Difluoroindole-2-carboxylic acid Use and application,4,6-Difluoroindole-2-carboxylic acid technical grade,usp/ep/jp grade.
Related News: “We believe the iLet Bionics Pancreas System represents a true breakthrough therapy for the management of glycemia, particularly in type 1 diabetes,” said Ed Damiano, President and CEO of Beta Bionics. “We are partuclarly excited by the possibility that the iLet may be able to provide safer and more effective therapy in far more people than current therapies due to its simplicity of use.”(4-fluorophenyl)-piperidin-4-ylmethanone,hydrochloride manufacturer “We believe the iLet Bionics Pancreas System represents a true breakthrough therapy for the management of glycemia, particularly in type 1 diabetes,” said Ed Damiano, President and CEO of Beta Bionics. “We are partuclarly excited by the possibility that the iLet may be able to provide safer and more effective therapy in far more people than current therapies due to its simplicity of use.”4-Hydroxybenzamide supplier At present, there are more than 8,000 domestic API manufacturers, but they mainly produce bulk APIs with low technical content.Diethyl 2-propyl-1H-imidazole-4,5-dicarboxylate vendor Advanced clinical trials with the Company’s lead compound, rigosertib, are aimed at what the Company believes are unmet medical needs of patients with MDS.Advanced clinical trials with the Company’s lead compound, rigosertib, are aimed at what the Company believes are unmet medical needs of patients with MDS.

