We serve 5-Bromo-2-fluoro-4-methylbenzaldehyde CAS:497224-12-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
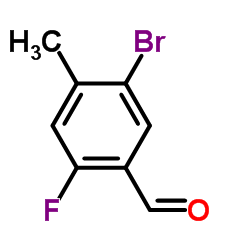
Contact us for information like 5-Bromo-2-fluoro-4-methylbenzaldehyde chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-bromo-6-fluoro-4-methylbenzaldehyde physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-Bromo-2-fluoro-4-methylbenzaldehyde Use and application,3-bromo-6-fluoro-4-methylbenzaldehyde technical grade,usp/ep/jp grade.
Related News: Under the terms of the agreement, ICIG will purchase substantially all of the pharmaceutical intermediates business, excluding the drug delivery technologies portion of the business.2-formylbenzoic acid manufacturer Here’s what we can tell you so far:2-Bromochlorobenzene supplier The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy.4-Chloro-2-fluorobenzoic acid vendor Citizens and residents will be allowed entry to New Zealand, but will be required to quarantine themselves for 14 days, Prime Minister Jacinda Ardern said.Under the sub-licensing agreement, Mankind will market the drug under its own trademark while Glenmark will manufacture and supply it to the drug firm.

