We serve 5-chlorouracil CAS:1820-81-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
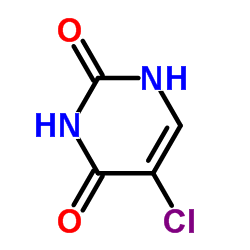
Contact us for information like 5-chloro-uracil chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,5-chloro-2,4-dioxopyrimidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-chloro-2,4-dioxopyrimidine Use and application,5-chloro-2,4-dioxopyrimidine technical grade,usp/ep/jp grade.
Related News: Italy and Vietnam included Taiwan in banning flights from China, a move that they announced after the W.H.O. declared the coronavirus outbreak a global health emergency.1-[(1,3,4-trimethyl-4,5-dihydroimidazol-1-ium-2-yl)oxy]pyrrolidine-2,5-dione,tetrafluoroborate manufacturer Most foreign drugmakers offered Beijing the lowest prices globally in a recent round of negotiations in order to get some of their new products included in national insurance program, a move that will help them gain access to more patients in less-affluent cities, officials said on Thursday.3,5-Dichloroaniline supplier Beneficial drug raw materials refer to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs). They are mainly used to meet the needs of original multinational pharmaceutical companies and emerging biopharmaceutical companies for innovative drugs in clinical drug research, registration approval and commercialization sales , Which also contains advanced intermediates used in the manufacture of the drug substance that need to be regulated by regulatory authorities.9-([1,1'-biphenyl]-3-yl)-3-bromo-9H-carbazole vendor The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.Such was the apparent demand sparked by the notice that the compound formula sold out on some stores on China’s e-commerce platform Taobao.

