We serve 6-Bromo-1-hexanol CAS:4286-55-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
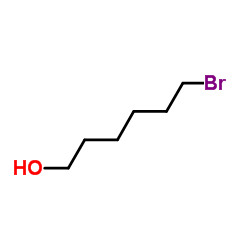
Contact us for information like 6-Bromo-1-Hexanol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,6-bromohexan-1-ol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-Bromo-1-Hexanol Use and application,6-Bromo-1-Hexanol technical grade,usp/ep/jp grade.
Related News: Thousands of cases have been identified in China, including in every province of the country.Thiophene-2-ethylamine manufacturer Citizens and residents will be allowed entry to New Zealand, but will be required to quarantine themselves for 14 days, Prime Minister Jacinda Ardern said.4-Amino-3-chlorophenol supplier What is the difference? Raw material refers to chemical compounds that are used as a base to make an API.4-(4-chlorophenyl)-N,N-diphenylaniline vendor What is the difference? Raw material refers to chemical compounds that are used as a base to make an API.DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.

