We serve 6-fluoro-2-methyl-3-nitropyridine CAS:18605-16-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
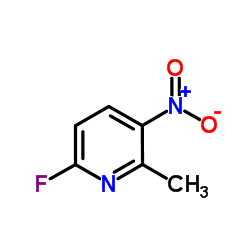
Contact us for information like 6-fluoro-2-methyl-3-nitropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Fluoro-5-nitro-6-methylpyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-fluoro-2-methyl-3-nitro-pyridine Use and application,2-Fluor-5-nitro-6-methylpyridin technical grade,usp/ep/jp grade.
Related News: This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?2-Bromoterephthalic acid manufacturer The purpose of APIs according to the FDA is to cause ‘pharmacological activity or other direct effects in the diagnosis, cure, mitigation, treatment or prevention of disease or to affect the structure and function of the human body.Pyridine-3-Sulfonyl Chloride supplier According to statistics, in the ten years from 2008 to 2017, a total of 13 “China Class 1” small-molecule chemical drugs were independently researched and developed by domestic enterprises in China and approved by the CFDA; in 2018, there were 10 varieties of domestic Class 1 new drugs. Approved for listing in China.3,4-Difluorobenzoic acid vendor Advanced clinical trials with the Company’s lead compound, rigosertib, are aimed at what the Company believes are unmet medical needs of patients with MDS.Raw material drug manufacturing is listed as one of the top ten key remediation industries, and it is necessary to implement clean transformation, new construction, reconstruction, and expansion of the ten key remediation industry construction projects to implement equal or reduced emissions of major pollutants; ) The industry implements the green enzyme production technology transformation; at the same time, the ten articles of water classify the industrial discharged sewage, and the sewage discharge of API companies is restricted.

