We serve 6-methyl-3-nitropyridin-2-amine CAS:21901-29-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
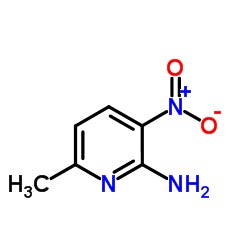
Contact us for information like 6-methyl-3-nitropyridin-2-amine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-amino-3-nitro-6-methylpyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-methyl-3-nitropyridin-2-amin Use and application,6-methyl-3-nitropyridin-2-amin technical grade,usp/ep/jp grade.
Related News: The Philippines, the United States and Australia have expanded travel restrictions, temporarily barring noncitizens who have recently traveled to China.D-serine manufacturer In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.3-Fluorobenzonitrile supplier According to statistics, in the ten years from 2008 to 2017, a total of 13 “China Class 1” small-molecule chemical drugs were independently researched and developed by domestic enterprises in China and approved by the CFDA; in 2018, there were 10 varieties of domestic Class 1 new drugs. Approved for listing in China.3-Hydroxypropionitrile vendor The policy-oriented emphasis on the medical and pharmaceutical industries and the direct support for the chemical raw material pharmaceutical industry have created a good political environment for the development of related enterprises and laid the foundation for the rapid development of the chemical raw material pharmaceutical industry. The good development prospects of the chemical raw material pharmaceutical industry will be passed directly to the upstream raw material industry, which will help stimulate the demand for the pharmaceutical intermediate market.The policy-oriented emphasis on the medical and pharmaceutical industries and the direct support for the chemical raw material pharmaceutical industry have created a good political environment for the development of related enterprises and laid the foundation for the rapid development of the chemical raw material pharmaceutical industry. The good development prospects of the chemical raw material pharmaceutical industry will be passed directly to the upstream raw material industry, which will help stimulate the demand for the pharmaceutical intermediate market.

