We serve 6-Oxo-1,6-dihydro-3-pyridazinecarboxamide CAS:60184-73-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
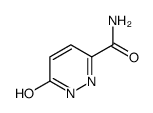
Contact us for information like 6-Oxo-1,6-dihydro-3-pyridazinecarboxamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,6-oxo-1,6-dihydro-pyridazine-3-carboxylic acid amide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-oxo-1,6-dihydropyridazine-3-carboxamide Use and application,6-oxo-1,6-dihydropyridazine-3-carboxamide technical grade,usp/ep/jp grade.
Related News: After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.4-Chloro-3-nitrobenzenesulfonamide manufacturer After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.(1R,2R)-(-)-1,2-Diaminocyclohexane supplier After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.2,6-Difluorophenol vendor We have leading expertise in strategy and operational implementation of pre-approval access programs making pharmaceutical products under clinical development available for patients and Inceptua’s clinical trial services business offers high quality clinical comparator sourcing and manufacturing services with an agile global supply chain to ensure that products are delivered exactly when needed.We have leading expertise in strategy and operational implementation of pre-approval access programs making pharmaceutical products under clinical development available for patients and Inceptua’s clinical trial services business offers high quality clinical comparator sourcing and manufacturing services with an agile global supply chain to ensure that products are delivered exactly when needed.

