We serve 7-Azabenzotriazol-1-Yloxytris(Dimethylamino)Phosphonium Hexafluorophosphate CAS:156311-85-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
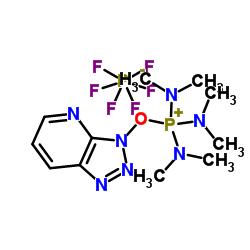
Contact us for information like 7-Azabenzotriazol-1-Yloxytris(Dimethylamino)Phosphonium Hexafluorophosphate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,AOP physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,AOP Use and application,AOP technical grade,usp/ep/jp grade.
Related News: Beneficial drug raw materials refer to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs). They are mainly used to meet the needs of original multinational pharmaceutical companies and emerging biopharmaceutical companies for innovative drugs in clinical drug research, registration approval and commercialization sales , Which also contains advanced intermediates used in the manufacture of the drug substance that need to be regulated by regulatory authorities.9-Bromo-1-nonanol manufacturer Beneficial drug raw materials refer to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs). They are mainly used to meet the needs of original multinational pharmaceutical companies and emerging biopharmaceutical companies for innovative drugs in clinical drug research, registration approval and commercialization sales , Which also contains advanced intermediates used in the manufacture of the drug substance that need to be regulated by regulatory authorities.3,3-diphenylpropanenitrile supplier Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.2-FLUORO-6-METHYLBENZONITRILE vendor Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.

