We serve 3-Bromopropionyl chloride CAS:15486-96-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
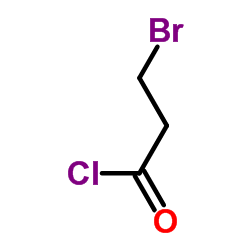
Chemical Name: 3-Bromopropionyl chloride
CAS.NO: 15486-96-1
Synonyms:
Propanoyl chloride, 3-bromo-
Molecular Formula: C3H4BrClO
Molecular Weight: 171.42000
Physical and Chemical Properties:
Density: 1.701 g / mL at 25 ° C (lit.)
Boiling point: 55-57 ° C17 mm Hg (lit.)
Flash point: 175 ° F
Refractive index: n20 / D 1.49 (lit.)
Specification:
Appearance: Clear yellow liquid
Purity:≥99.0%
Heavy metal:≤ 0.1%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:Keep away from heat, sparks, and flame. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Application:Pharmaceutical Intermediate.
Contact us for information like 3-Bromopropionyl chloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Propanoyl chloride, 3-bromo- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Bromopropionyl chloride Use and application,3-Bromopropionyl chloride technical grade,usp/ep/jp grade.
Related News: Health officials said it remained unclear where he had developed the disease.4H-3,1-Benzoxazine-2,4(1H)-dione manufacturer Health officials said it remained unclear where he had developed the disease.4-Chloro-2-methylpyridine supplier Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.2-Aminobenzenethiol vendor Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.“With early evidence of clinical activity for our off-the-shelf, iPSC-derived NK cell programs, we are excited to lead in bringing next-generation CAR T-cell therapies to patients and plan to submit an IND for FT819 in the first half of 2020.”

