We serve Fmoc-5-aminopentanoic acid CAS:123622-48-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
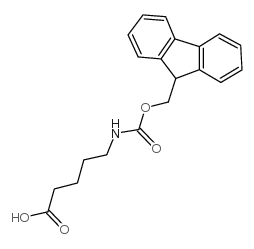
Chemical Name: Fmoc-5-aminopentanoic acid
CAS.NO: 123622-48-0
Molecular Formula:C20H21NO4
Molecular Weight: 339.38500
Synonyms:
Fmoc-5-Ava-OH
5-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)pentanoic acid
FMOC-5-AMINOPENTANOIC ACID
Physical and Chemical Properties:
Density: 1.231g / cm3
Boiling point: 562.24ºC at 760 mmHg
Flash point: 293.832ºC
Refractive index:/
Specification:
Appearance: White Crystalline Powder
Purity:≥98%
Moisture Content: ≤0.01%
Loss On Drying: ≤0.2%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:Store at 2-8ºC.Keep the container tightly closed, put it in a tight dispenser, and store in a cool, dry place.
Application:pharmaceutical intermediates.
Contact us for information like Fmoc-5-aminopentanoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,FMOC-5-AMINOPENTANOIC ACID physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,5-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)pentanoic acid Use and application,5-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)pentanoic acid technical grade,usp/ep/jp grade.
Related News: In order to find answers to these questions, our staff in the development department set about conducting a series of experiments.N-Cbz-glycine Ethyl Ester manufacturer From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.4-Aminobenzonitrile supplier Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.2-Methylbenzotrifluoride vendor Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.Profitability continued to improve. The company’s comprehensive gross profit margin for the first three quarters was 36.06%, a year-on-year increase of 3.44 percentage points. Since this year, the company’s profitability has remained at a relatively high level. On the one hand, due to the decline in the trading business with lower profitability, the proportion has declined.

