We serve 3,5-Dimethylbenzonitrile CAS:22445-42-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
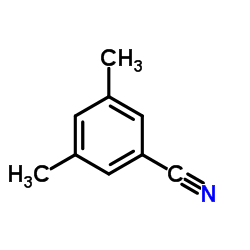
Contact us for information like 3,5-Dimethylbenzonitrile chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3,5-dimethyl benzonitrile physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3,5-dimethyl benzonitrile Use and application,3,5-Dimethylbenzonitrile technical grade,usp/ep/jp grade.
Related News: Others — somewhat more cynically — wondered if this was a concerted effort to promote certain herbal products to boost their makers’ share prices ahead of the Chinese stock market’s re-opening Monday.2,3-Difluoro-5-methylbenzoic acid manufacturer In order to find answers to these questions, our staff in the development department set about conducting a series of experiments.2-FLUORO-5-HYDROXYPYRIDINE supplier In order to find answers to these questions, our staff in the development department set about conducting a series of experiments.4,4'-Diiodobiphenyl vendor Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

