We serve Chemical Name:6-nitrosothymol CAS:2364-54-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
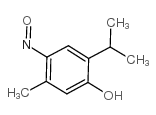
Chemical Name:6-nitrosothymol
CAS.NO:2364-54-7
Synonyms:5-methyl-4-nitroso-2-propan-2-ylphenol;5-methyl-2-(1-methylethyl)-4-nitroso-phenol;2-ISOPROPYL-5-METHYL-4-NITROSOPHENOL;Nitrosothymol;4-nitrosothymol;2-iso-propyl-5-methyl-4-nitrosophenol;5-METHYL-4-NITROSO-2-ISOPROPYLPHENOL;PARA-NITROSOTHYMOL;6-Nitroso-3-oxy-1-methyl-4-isopropyl-benzol;p-nitrosothymol;Thymol,6-nitroso
Molecular Formula:C10H13NO2
Molecular Weight:179.21600
HS Code:2908999090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:318.8ºC at 760 mmHg
Density:1.1g/cm3
Index of Refraction:1.534
PSA:49.66000
Exact Mass:179.09500
LogP:3.22190
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 5-methyl-4-nitroso-2-propan-2-ylphenol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Thymol,6-nitroso physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-Nitroso-3-oxy-1-methyl-4-isopropyl-benzol Use and application,2-ISOPROPYL-5-METHYL-4-NITROSOPHENOL technical grade,usp/ep/jp grade.
Related News: Under the strict supervision of environmental protection, it has helped the API industry to continuously optimize its product structure, realize industrial upgrading, and transition to the development of high-end APIs. 6-nitrosothymol manufacturer For example, there are concerns that PFAS’ effect on the thyroid could lead to thyroid cancer, van Gerwen said. 6-nitrosothymol supplier ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment��s chemistry, manufacturing and controls process, but not its safety. 6-nitrosothymol vendor The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation. 6-nitrosothymol factory FT was defined as any difficulty paying medical bills, high financial distress, cost-related medication nonadherence, food insecurity, and/or foregone/delayed care due to cost.

