We serve Chemical Name:ribostamycin CAS:25546-65-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
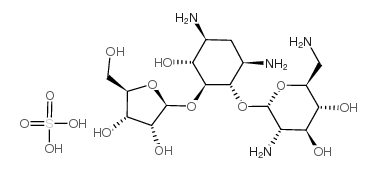
Chemical Name:ribostamycin
CAS.NO:25546-65-0
Synonyms:Ribastamin;Antibiotic SF 733;Ribostamicina;Ribostamycinum;(2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxycyclohexyl]oxyoxane-3,4-diol;Vistamycin;Xylostatin;Hetangmycin;Dekamycin IV;Ribostamycine
Molecular Formula:C17H36N4O14S
Molecular Weight:552.55100
HS Code:
Physical and Chemical Properties:
Melting point:192-195°; mp 175-180° (dec)
Boiling point:N/A
Density:N/A
Index of Refraction:1.662
PSA:345.36000
Exact Mass:552.19500
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Ribastamin chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ribostamycine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Antibiotic SF 733 Use and application,(2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxycyclohexyl]oxyoxane-3,4-diol technical grade,usp/ep/jp grade.
Related News: After this long manufacturing process, it is purified until it reaches a very high degree of purity and finally becomes an API. ribostamycin manufacturer ��The number of confirmed cases of coronavirus in Taiwan is not higher than in most countries affected,�� Mr. Wu said. ��Other than China, no other country, no other country has had its flight banned by Italy.�� ribostamycin supplier High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies. ribostamycin vendor After this long manufacturing process, it is purified until it reaches a very high degree of purity and finally becomes an API. ribostamycin factory With nearly 194 billion US dollars of original research medicines facing the expiration of patents in the past five years, more and more domestic companies are focusing on the corresponding characteristic APIs, and have started research and development and production preparations in advance. The scale of production and export of APIs will continue to expand and grow.

