We serve Chemical Name:1-Mesitylmethanamine CAS:40393-99-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
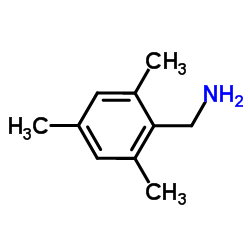
Chemical Name:1-Mesitylmethanamine
CAS.NO:40393-99-5
Synonyms:1-(2,4,6-trimethylphenyl)methanamine;Benzenemethanamine, 2,4,6-trimethyl-;1-Mesitylmethanamine;(2,4,6-trimethylphenyl)methanamine;Mesitylmethanamine
Molecular Formula:C10H15N
Molecular Weight:149.233
HS Code:2921199090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:246.5±9.0 °C at 760 mmHg
Density:0.9±0.1 g/cm3
Index of Refraction:1.534
PSA:26.02000
Exact Mass:149.120453
LogP:2.47
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1-(2,4,6-trimethylphenyl)methanamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Mesitylmethanamine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2,4,6-trimethylphenyl)methanamine Use and application,(2,4,6-trimethylphenyl)methanamine technical grade,usp/ep/jp grade.
Related News: Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK. 1-Mesitylmethanamine manufacturer The mode of cooperation between domestic API companies and large international companies can lower the export threshold. 1-Mesitylmethanamine supplier The company’s pharmaceutical intermediate business is expected to maintain a growth rate of more than 30%. 1-Mesitylmethanamine vendor Lilly has denied retaliating against any employees, and no lawsuit has been filed on behalf of Mula. 1-Mesitylmethanamine factory From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.

