We serve Chemical Name:Allopurinol riboside CAS:16220-07-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
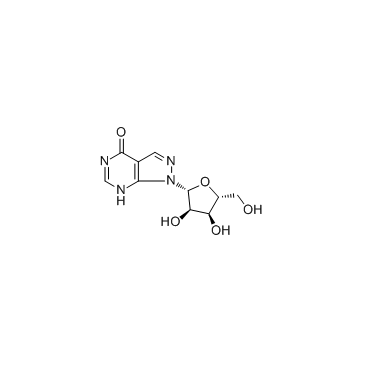
Chemical Name:Allopurinol riboside
CAS.NO:16220-07-8
Synonyms:8-Aza-6-hydroxy-7-deazapurine riboside;Allopurinol ribonucleoside;8-AZA-7-DEAZAINOSINE;<4-Hydroxy-1H-pyrazolo<3,4-d>pyrimidin>-ribosid;Allopurinol-1-ribonucleoside
Molecular Formula:C10H12N4O5
Molecular Weight:268.22600
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:570.9ºC at 760mmHg
Density:2.08g/cm3
Index of Refraction:1.925
PSA:133.49000
Exact Mass:268.08100
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like 8-Aza-6-hydroxy-7-deazapurine riboside chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Allopurinol-1-ribonucleoside physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,8-Aza-6-hydroxy-7-deazapurine riboside Use and application,-ribosid technical grade,usp/ep/jp grade.
Related News: Complete the research and development of APIs and intermediates, design new patented process routes, and explore new crystal forms. Allopurinol riboside manufacturer Vietnam backtracked on Saturday and narrowed its restrictions to most flights from mainland China. Allopurinol riboside supplier Vietnam backtracked on Saturday and narrowed its restrictions to most flights from mainland China. Allopurinol riboside vendor At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world. Allopurinol riboside factory At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world.

