We serve 2-Fluoro-4-iodobenzonitrile CAS:137553-42-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
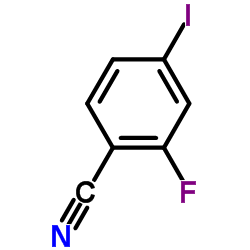
Contact us for information like 2-Fluoro-4-iodobenzonitrile chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Fluoro-4-iodobenzonitrile physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Fluoro-4-iodobenzonitrile Use and application,2-Fluoro-4-iodobenzonitrile technical grade,usp/ep/jp grade.
Related News: Professor Coleman told Sky News: “Unfortunately there is no vaccine and no specific therapeutics that can be used against the coronavirus.L-tert-Leucine manufacturer “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.3-chloro-2-methylpropan-1-ol supplier “Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations” (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.1-PBFR vendor Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers.In these situations, the API is not a single substance but the culmination of various ingredients.

